The Many SHTF Uses for Alcohol

Ethanol (C2H5OH) is the common form of alcohol that you consume as a beverage/within a beverage. There are different percentages of alcohol per different beverages. Here are some rough “guidelines” of the percentages:
Vodka (usually 40%), Brandy (usually 40%), Scotch (40-60%), Grain alcohol (85-95%), Gin (37-60%).
Methanol is also known as wood alcohol (CH3OH). As the name implies, it is taken from wood, and its main use is in industry, and in high-performance engines such as racecars and “monster” trucks, as well as other specialty engines. Methanol does not give a flame off when burning and can be put out with water.
Isopropyl alcohol (also called “rubbing” alcohol) sees a use in several different household needs from cleaning to disinfecting. Isopropyl (C3H8O) alcohol is widely available in all your grocery and big-box stores and varies in concentration from about 50% all the way up to 99% (usually found in feed stores or hardware stores in that concentration).
Now for winter considerations, here is an important chart for you that lists the freezing points (the point of transition between the liquid becoming a solid and vice-versa) of alcohols:
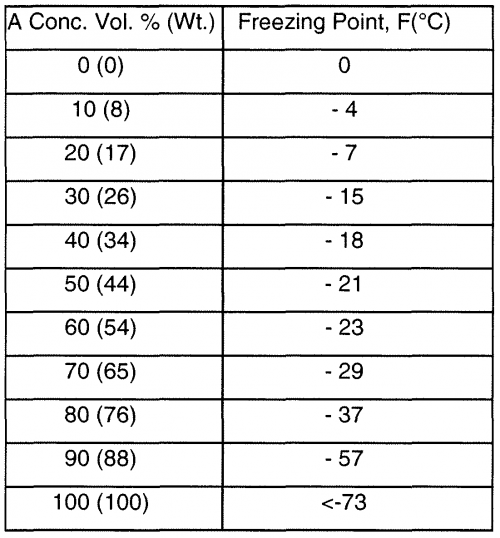
Source: http://patentimages.storage.googleapis.com/WO2010029344A2/imgf000023_0001.png
We must keep in mind that this is primarily used for ethanol, the type that is consumed as a beverage. I’m not listing freezing points of the other two types for a reason: you can’t drink them or consume them. Hear me out, as I give you the main point:
…click on the above link to read the rest of the article…